MR-Linac: Advances in Radiation Therapy

A brief overview of advances in MRI-linear accelerator systems for radiation therapy treatment, covering everything from how they work, to their advantages of CBCT, and disadvantages of MRI-linac systems.

It is critical for RTTs to keep up with advances in technology in the field in order to provide the best quality care to patients. In this article, we introduce the MR-linac, a powerful tool that has allowed for unprecedented precision in delivering radiation therapy treatments. Below we’ll cover what an MRI linac is, how it is used in radiation therapy, how it works, what it adds to the field, and some of its disadvantages.
- What is an MR-linac?
- How do MR-linacs work?
- A Brief History of MRI and Linear Accelerator Development
- The Need for MR-Linac Development
- MR-Linac Advances
- Disadvantages
- Future Development
- Concluding Thoughts
What is an MR-linac?
The MR-linac, or MRI-linac, combines two machines that had previously only been used independently in radiation therapy: a high-strength magnetic resonance imaging (MRI) machine and a linear accelerator. The combination of these two machines into a single tool enables greater precision in cancer treatments.
How do MR-linacs work?
In brief, while the MRI machine produces high-quality diagnostic images of a tumor in real time, the linear accelerator maintains a sharp focus, targeting the tumor with high-energy beams, allowing for much greater precision in treatment delivery.
A Brief History of MRI and Linear Accelerator Development
In the past century, external radiation therapy has become increasingly technically sophisticated. This technical advancement began following the discoveries of X-rays by Rœntgen in 1895, and radioactivity by Becquerel in 1896. In the late 1960s, the first linear electron accelerators with great collimation appeared following the arrival of the multi-leaf collimator in 1965, which resulted in increasingly precise beams, allowing considerable targeting of the volumes to be treated, and the protection of organs at risk.
In 1974, computerized tomography appeared. This led to another great revolution in the field with the birth of 3D conformal radiation therapy. Today, image-guided radiation therapy has increased the efficiency of treatments for cancer patients by tracking tumor responses with greater accuracy, which in turn allows for treatments to be adjusted accordingly. While there are several imaging techniques available, only a few have been shown to be helpful for guiding radiation therapy. MR imaging in particular has recently gained a lot of attention as a potential solution for providing real-time imagery for truly adaptive radiation therapy.
The gist: As imaging technology has advanced, several techniques have been developed that allow for greater tumor tracking and treatment adjustment, but MRI has gained the most attention for its ability to provide real-time imagery which allows for more truly adaptive radiation therapy
The Need for MR-Linac Development
- CBCT images do not offer high-quality soft tissue contrast, making it extremely difficult to visualize tumors in areas such as the brain and liver. In other words, even if the tumor can be seen, it is difficult to distinguish cancerous tissue from surrounding healthy tissue
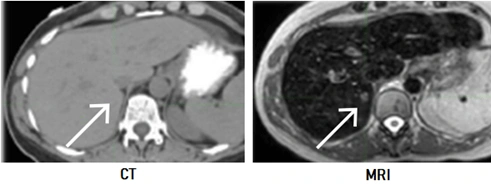
- Visualization is also impaired in regions with continuous motion, such as breathing or swallowing
- It subjects the patient to additional radiation exposure and treatment progress cannot be verified as frequently
It is for these reasons that extensive research is being conducted on how MRI scans can be integrated into the treatment planning and verification process: MRI improves soft tissue contrast and potentially eliminates the motion problem by using respiratory gating, which continuously monitors the breathing cycle and captures the image at optimal point in the cycle.
The gist: The MRI-linac was born out of the need for improved visualization of specific tissues, to address issues of natural patient movement, and greater precision in tumor targeting, all of which were not possible through cone beam CT (CBCT)
MR-Linac Advances
During radiation therapy treatment, a patient’s tumor may shift about as he or she breathes. The tumor can sometimes move around in by more than an inch (more than 2.54 cm). Due to this natural movement, keeping the radiation beams precisely focused on the tumor during the treatment session may prove difficult. Before MR-linac, to ensure that the whole tumor was treated, radiation oncologists would have to treat the entire area into which the tumor could move as the patient breathed normally.
Now, however, thanks to the combined powers of MRI and a linear accelerator, this is no longer the case. The MR-linac continuously adjusts the radiation delivery to compensate for any shifts in the tumor’s position by monitoring its movement in real time. The MR-linac also keeps track of a tumor’s position and the soft tissues around it from one treatment session to the next, adjusting the radiation therapy delivery as needed to account for any changes.
MR-linac has prove particularly useful for treating tumors that shift location from one day to the next as a result of the movement of adjacent organs, such as lung tumors that move each time a patient takes a breath and tumors in the abdomen, which can move depending on the bowel or bladder capacity of the patient.
For example, with stereotactic ablative radiation, doctors can now control over 90% of early-stage lung cancers. And with the MR-linac permitting the tracking of tumor movement with each breath, radiation oncologists can improve their success rate even. Knowing where the tumor is during therapy allows them to deliver radiation only when it is in the target field.
The gist: MRI-linac tracks tumor movement in real time and adjusts radiation delivery to compensate for shifts in tumor location on the spot. It even tracks tumor and soft tissue location from one session to the next.
The MRI-linear accelerator combo could help radiation oncologists overcome a number of problems. One of the most difficult problems is predicting when a healthy organ will demonstrate considerable radiation toxicity.
One explanation for this is that humans differ in their sensitivity to radiation. Some patients simply have higher sensitivity than others. Another difficulty is that we have no idea how much healthy tissue has been exposed to radiation during treatment—we simply don’t have a reliable way to keep track of it.
One of the most important benefits of having superior soft tissue imaging during treatment is that it allows us to target and measure the cumulative dose to both tumor and healthy tissues over time.
The combination MRI and linear accelerator system also enables radiation oncology to be adjusted to the patient’s tumor. The majority of radiation treatment consists of creating a plan ahead of time and delivering that exact same plan without modification.
The MRI-linear accelerator gives the option of adjusting the radiation therapy treatment as you go along based on tumor response. It has the potential to provide useful information about a patient’s particular cancer biology, as well as the ability to personalize treatment rather than using one-size-fits-all treatments. We can really see the response as it happens during their treatment course and make adjustments if needed, thanks to high-quality imaging conducted on a daily basis.
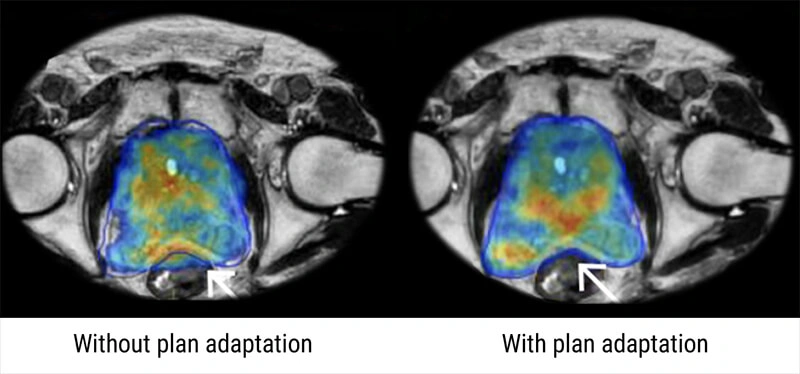
Oncologists may also be able to decide if a patient needs more therapy in the future using MRI-linear accelerator systems based on the patient’s in-treatment imaging response. Some patients, for example, may have such a positive response to treatment that they do not require surgery, whereas those whose tumors do not respond well may require more invasive surgery or chemotherapy as a follow-up.
The gist: MRI-linac can potentially solve the issues of individual patient’s varying sensitivity to radiation, as well as allow for personalized treatment, specifically tailored to the patient’s unique tumor and cancer biology
MR-linac technology is particularly appropriate for treating tumors that are adjacent to critical sensitive structures, such as those found on the pancreas. The small intestine, stomach, kidneys, and spinal cord are all in close proximity to the pancreas. Many cancers are located near organs that cannot withstand high radiation doses, such as the intestines or stomach. As a result, the radiation doses we want to provide are constrained by our capacity to avoid damaging vital or nearby organs.
For a variety of reasons, the use of combination MRI-linac system may allow for the delivery of a higher radiation dose, if it is required. With MR-linac, doctors don’t have to worry about delivering such high dosages to healthy tissue, because they can tell when it is and isn’t in the way. And if doctors can see the intestine right next to the tumor and know it isn’t moving into the field, they can give a considerably higher dose of radiation to the tumor.
The gist: Because MRI-linear accelerator systems allow for accurate tracking of tumor movement, it is particularly helpful in treating tumors that are near sensitive and critical structures. It allows for us to avoid delivering radiation to unaffected organs, and subsequently, allows for the delivery of higher doses to the tumor, if necessary.
Disadvantages
While there are many benefits to merging MRI with linear accelerators, there are also some drawbacks. One downside to MRI-linac is that it is expected to be more expensive than regular radiation therapy. When compared to chemotherapy, surgery, or other cancer treatments, radiotherapy is quite affordable. As a result of its efficiency, radiotherapy is more affordable. Using the MR-linac will very certainly be more costly, and reimbursement may also be a problem.
Time could be an issue, as well, albeit a smaller one. A standard MR-linac session should not take any longer than a traditional linear accelerator session. The use of diffusion weighted imaging to detect tumor response, on the other hand, could lengthen treatment sessions. Radiation treatments usually last 15 to 20 minutes. Diffusion-weighted imaging can add five to ten minutes to the procedure. So, we are not talking about a very significant increase in the amount of time, especially compared to the potential benefit.
MR-linac would also be an unavailable treatment option for a certain portion of patients. Certain individuals with metal implants are unable to have MRIs. Safety in the MR environment is also a concern. While MR is nonionizing and poses no risk of exposing patients to excessive radiation, a strong magnet necessitates the implementation of proper safety protocols. At the same time, the requisite MRI competence, knowledge, and the necessity for on-the-spot decision making are considered as a disadvantage.
To ensure that MRI-linac is utilized properly for radiation therapy and is safe for both patients and users, adequate training programs are required. In a study of implementing MRI-linac systems (Hehakaya et al., 2020), several respondents found it necessary to expand the roles RTTs in order to reduce the presence of radiation oncologists and physicists during treatment sessions, thereby reducing personnel costs. The authors of the study noted, however, that while radiation technicians could assume more responsibilities, their limited availability and the fact that they are not able to independently approve treatment plans is problematic.
The gist: Among the disadvantages of MRI-linac are increased cost, slight increases in session times, MR safety, and training for staff related to MR-linac usage.
Future Development
Physicians can see how well a treatment is working as they deliver it, if they can image the patient during treatment rather than before and after. If the tumor isn’t responding, they may need to increase the dose. Or they may need to reduce the dose or number of treatments if the tumor is responding effectively after only a few of the planned courses of treatment. Radiation oncologists must usually wait three months or longer after treatment to reimage their patients and assess their response.
In the future, clinicians will be able to use MRI diffusion weighted imaging to assess the efficacy of a treatment as it is delivered. There are several things that MR sequences can show you, and one of them is water diffusion in tissue. At the cellular level, this can tell you how densely packed the tissues are. Tissue death is indicated by the ability of water to diffuse more readily between cells. We wouldn’t have been able to pick up on that before.
In a pilot study published in Medical Physics (March 2016), researchers at the University of California, Los Angeles executed a longitudinal diffusion MR strategy to assess patient response to radiation therapy using ViewRay’s MRI-guided radiotherapy equipment. They concluded that this approach could potential enable response-guided adaptive radiotherapy, but that additional research is needed.
Based on their expertise with the combination machine, researchers want to eventually automate the optimum sequences for different types of cancers and treatments. MR imaging could aid in the identification of organs and their parts, for example. With that information, we may discover that one sequence is required for a pancreatic tumor that is adjacent to the duodenum and another for a tumor that is mid-pancreas and not consistently adjacent to the bowel. It’s not a question of whether or not MR images can be used—it’s merely a matter of putting these sequences together.
Concluding Thoughts
Because of its unique imaging qualities of enhanced soft-tissue contrast, functional information, and real-time imaging without radiation dose, the use of MRI in the radiation therapy workflow for simulation and delivery guidance is rapidly growing. However, one of the most difficult aspects of bringing MRI into the RT environment is ensuring MR safety. The majority of RT employees aren’t used to working in a high magnetic field environment.
But the diagnostic imaging community has a wealth of information and expertise on MR safety, and with collaboration and cross-training great strides can be made to expanding the use of MR-linac systems.
References
- Brigham and Women’s Hospital. Advanced radiation treatment with MRI-guided linear accelerator (MRI-LINAC). https://www.brighamandwomens.org
- Elekta. (2020). Elekta Unity [Brochure].
- Elekta. (2021). Elekta unity. https://www.elekta.com
- Freeman, T. (2020, December 14). Is the MR-linac the future of adaptive radiotherapy? Physics World. https://physicsworld.com
- Hehakaya, C., Van der Voort van Zyp, J.R., Lagendijk. J.J.W, Grobbee, D.E., Verkooijen, H.M., & Moors, E.H.M. (2020) Problems and promises of introducing the magnetic resonance imaging linear accelerator into routine care: The case of prostate cancer. Frontiers in Oncology, 10, 1741. doi: 10.3389/fonc.2020.01741
- Hu, Q., Yu, V. Y., Yang, Y., Hu, P., Sheng, K., Lee, P. P., Kishan, A. U., Raldow, A. C., O’Connell, D. P., Woods, K. E., & Cao, M. (2020). Practical Safety Considerations for Integration of Magnetic Resonance Imaging in Radiation Therapy. Practical Radiation Oncology, 10(6), 443–453. https://doi.org
- Moffit Cancer Center. MRI-linac (MRI-guided adaptive radiation therapy. https://moffitt.org
- Orenstein, B.W. (2017). Real-time radiation therapy. Radiology Today, 18(1), 16. https://www.radiologytoday.net
- The Royal Marsden: NHS Trust Foundation. The UK’s first MR linac, combining MRI and radiotherapy technology. https://www.royalmarsden.nhs.uk
- Winkel, D., Bol, G. H., Kroon, P. S., van Asselen, B., Hackett, S. S., Werensteijn-Honingh, A. M., Intven, M., Eppinga, W., Tijssen, R., Kerkmeijer, L., de Boer, H., Mook, S., Meijer, G. J., Hes, J., Willemsen-Bosman, M., de Groot-van Breugel, E. N., Jürgenliemk-Schulz, I. M., & Raaymakers, B. W. (2019). Adaptive radiotherapy: The Elekta Unity MR-linac concept. Clinical and Translational Radiation Oncology, 18, 54–59. https://doi.org
Disclaimer: The information provided on this website is intended to provide useful information to radiologic technologists. This information should not replace information provided by state, federal, or professional regulatory and authoritative bodies in the radiological technology industry. While Medical Professionals strives to always provide up-to-date and accurate information, laws, regulations, statutes, rules, and requirements may vary from one state to another and may change. Use of this information is entirely voluntary, and users should always refer to official regulatory bodies before acting on information. Users assume the entire risk as to the results of using the information provided, and in no event shall Medical Professionals be held liable for any direct, consequential, incidental or indirect damages suffered in the course of using the information provided. Medical Professionals hereby disclaims any responsibility for the consequences of any action(s) taken by any user as a result of using the information provided. Users hereby agree not to take action against, or seek to hold, or hold liable, Medical Professionals for the user’s use of the information provided.
