Breast Cancer Screening: Advances in Imaging Technology
Cutting-Edge Advancements in Breast Cancer Screening: What You Need to Know
Introduction
As breast cancer screening continues to evolve, innovations are improving early cancer detection, diagnostic accuracy, and patient care. Here’s a breakdown of some of the most recent advancements that are making waves in breast imaging, designed for the everyday practice of radiologic technologists (rad techs) and sonographers.
Digital Breast Tomosynthesis (DBT)
DBT, also known as 3D mammography, has become a game-changer in breast cancer screening. By capturing multiple images of the breast from different angles, DBT reconstructs a layered, three-dimensional view. This technology is particularly helpful in diagnosing invasive cancers in women with dense breast tissue, reducing false positives and follow-up testing.
DBT gives you a clearer, more detailed view, improving diagnostic accuracy and helping you spot cancers earlier than traditional 2D mammography.

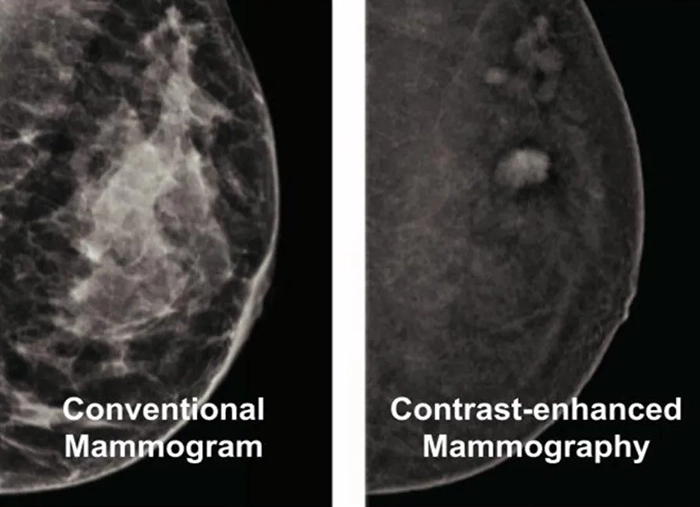
Contrast-Enhanced Mammography (CEM)
Combining the principles of mammography with the use of a contrast agent, CEM highlights areas with increased blood flow—often a marker for malignancies. CEM can reveal hidden tumors in dense breasts and provides an affordable alternative to MRI for those who cannot undergo it.
CEM is a cost-effective, reliable option for detecting cancer in patients where MRI is not viable, giving you more diagnostic tools at your disposal.
https://www.breastimagingvictoria.com.au/
Artificial Intelligence (AI) in Mammography
AI is making its mark on breast cancer screening and imaging by assisting rad techs and radiologists in analyzing mammograms. AI tools scan mammograms for abnormalities, flagging potential issues that could be missed by the human eye. This can lead to quicker reads, fewer errors, and enhanced workflow efficiency.
AI won’t replace you, but it will help you work more efficiently and improve diagnostic accuracy, allowing you to focus on patient care and complex cases.
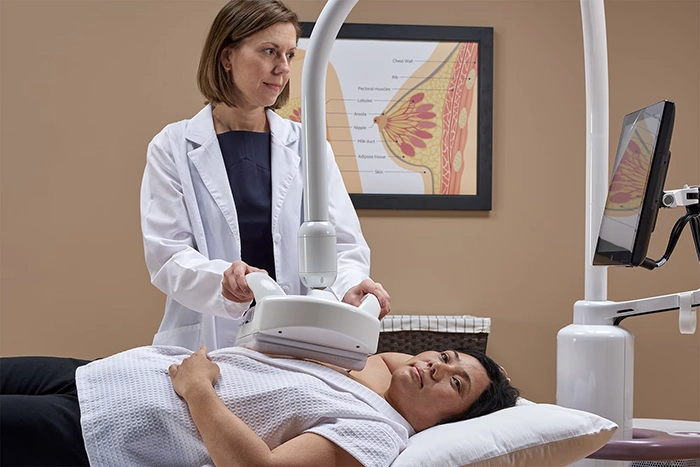
Automated Breast Ultrasound (ABUS)
ABUS is designed to supplement mammography, particularly in women with dense breast tissue, which can obscure cancers. Unlike handheld ultrasound, ABUS is fully automated, reducing operator variability and ensuring consistent, comprehensive breast scans.
ABUS standardizes the imaging process and provides better coverage of dense breasts, giving you peace of mind with less chance of missing a hidden cancer.
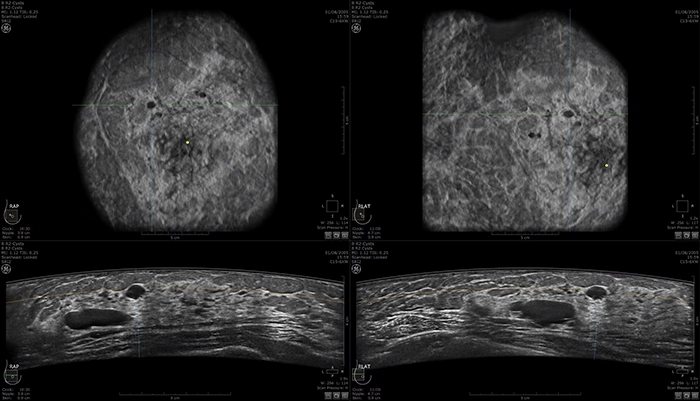
Case of multiple cysts on ABUS images: https://www.gehealthcare.com/
Molecular Breast Imaging (MBI)
MBI offers a functional look at the breast by using a radiotracer to detect cancerous cells based on their metabolic activity. It’s proving to be particularly useful in women with dense breast tissue, where traditional imaging might fall short.
MBI adds a functional layer to breast cancer screening and imaging, helping detect smaller tumors that could otherwise be missed, making it an invaluable tool for high-risk patients.
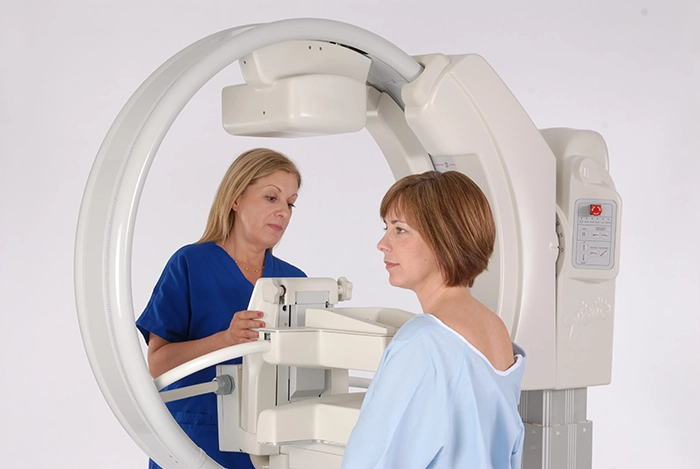
Elastography
Elastography uses ultrasound to measure tissue stiffness, offering additional information beyond traditional grayscale ultrasound images. Cancerous tissue tends to be stiffer than benign tissue, allowing elastography to help differentiate between the two. Adding elastography to your toolbox allows for a more detailed characterization of breast lesions, improving your ability to distinguish benign from malignant masses.

https://www.siemens-healthineers.com/
Siemens Healthineers ultrasound next-generation 2D-Shear Wave Elastography (2D SWE) updated algorithm adapts to better characterizing stiff lesions providing improved performance in the delineation of small and stiff lesions, previously identified as blue cancers.
High-Resolution Breast MRI
MRI continues to be a critical tool for high-risk patients, but recent advancements in image resolution and faster scan sequences are taking it to the next level. Enhanced imaging techniques like diffusion-weighted imaging (DWI) are making MRI more effective in detecting small lesions.
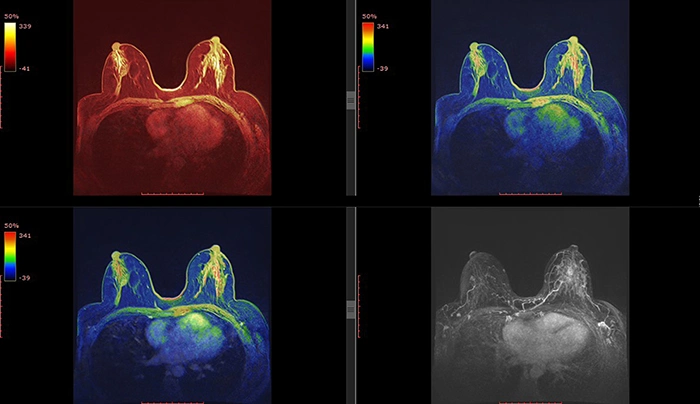
New MRI technologies are improving breast cancer screening and detection in high-risk populations, offering a more detailed view, especially when dense tissue or prior scarring makes other imaging difficult.
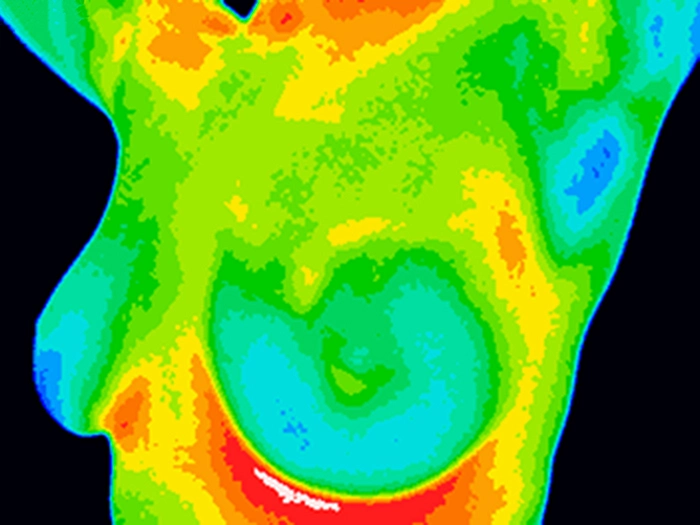
Thermography
Although still in its infancy, thermography detects heat patterns in breast tissue to identify areas of abnormal blood flow, a potential sign of cancer. While it’s not widely accepted yet, ongoing research aims to improve its accuracy as a non-invasive, radiation-free screening tool.
Keep an eye on thermography as research evolves. It may eventually provide a supplementary, radiation-free breast cancer screening method for certain patient populations.
Conclusion
These cutting-edge advancements in breast cancer screening and imaging represent new opportunities to improve the accuracy and efficiency of breast cancer detection. Whether you’re working with dense tissue, improving workflow through AI, or exploring new imaging techniques, staying up-to-date on these technologies will help you provide the best care possible for your patients.
References
- Conant, E. F., et al. (2016). “Digital breast tomosynthesis: a clinical review.” European Journal of Radiology, 82(9), 2003-2009.
- Freer, P. E. (2015). “Digital breast tomosynthesis.” Radiologic Clinics of North America, 52(3), 489-497.
- Jochelson, M. S., et al. (2013). “Contrast-enhanced digital mammography: a pilot study.” American Journal of Roentgenology, 202(2), 360-366.
- Lobbes, M. B. I., et al. (2017). “Contrast-enhanced mammography: techniques, current results, and potential indications.” Clinical Radiology, 68(9), 935-944.
- Rodríguez-Ruiz, A., et al. (2019). “Detection of breast cancer with mammography: Effect of an artificial intelligence support system.” Radiology, 290(3), 305-314.
- McKinney, S. M., et al. (2020). “International evaluation of an AI system for breast cancer screening.” Nature, 577(7788), 89-94.
- Skaane, P. (2018). “Automated breast ultrasound (ABUS) in mammography screening of women with dense breasts.” Acta Radiologica, 59(7), 747-752.
- Vourtsis, A. (2019). “Three-dimensional automated breast ultrasound: technical aspects and first results.” Diagnostic and Interventional Imaging, 100(5), 243-254.
- Hruska, C. B., & O’Connor, M. K. (2013). “Molecular breast imaging for screening in dense breasts and its potential impact on breast cancer mortality.” Breast Cancer Research and Treatment, 138(1), 257-262.
- Rhodes, D. J., et al. (2015). “Molecular breast imaging at reduced radiation dose for supplemental screening in mammographically dense breasts.” American Journal of Roentgenology, 204(2), 241-251.
- Goddi, A., et al. (2012). “Breast elastography: a literature review.” Journal of Ultrasound, 15(3), 192-198.
- Barr, R. G. (2017). “Breast elastography: past, present, and future.” Seminars in Ultrasound, CT and MRI, 38(1), 87-97.
- Mann, R. M., et al. (2019). “Breast MRI: state of the art.” Radiology, 292(3), 520-536.
- Comstock, C. E., et al. (2017). “Diffusion-weighted MRI for breast cancer detection and diagnosis.” Radiology, 284(2), 360-370.
- Head, J. F., et al. (2000). “Infrared imaging of the breast: initial reappraisal using high-resolution digital technology in 100 successive cases of stage I and II breast cancer.” American Journal of Roentgenology, 180(1), 263-269.
- Gautherie, M. (1980). “Thermopathology of breast cancer: measurement and analysis of in vivo temperature and blood flow.” Annals of the New York Academy of Sciences, 335(1), 383-415.
Disclaimer: The information provided on this website is intended to provide useful information to radiologic technologists. This information should not replace information provided by state, federal, or professional regulatory and authoritative bodies in the radiological technology industry. While Medical Professionals strives to always provide up-to-date and accurate information, laws, regulations, statutes, rules, and requirements may vary from one state to another and may change. Use of this information is entirely voluntary, and users should always refer to official regulatory bodies before acting on information. Users assume the entire risk as to the results of using the information provided, and in no event shall Medical Professionals be held liable for any direct, consequential, incidental or indirect damages suffered in the course of using the information provided. Medical Professionals hereby disclaims any responsibility for the consequences of any action(s) taken by any user as a result of using the information provided. Users hereby agree not to take action against, or seek to hold, or hold liable, Medical Professionals for the user’s use of the information provided.
